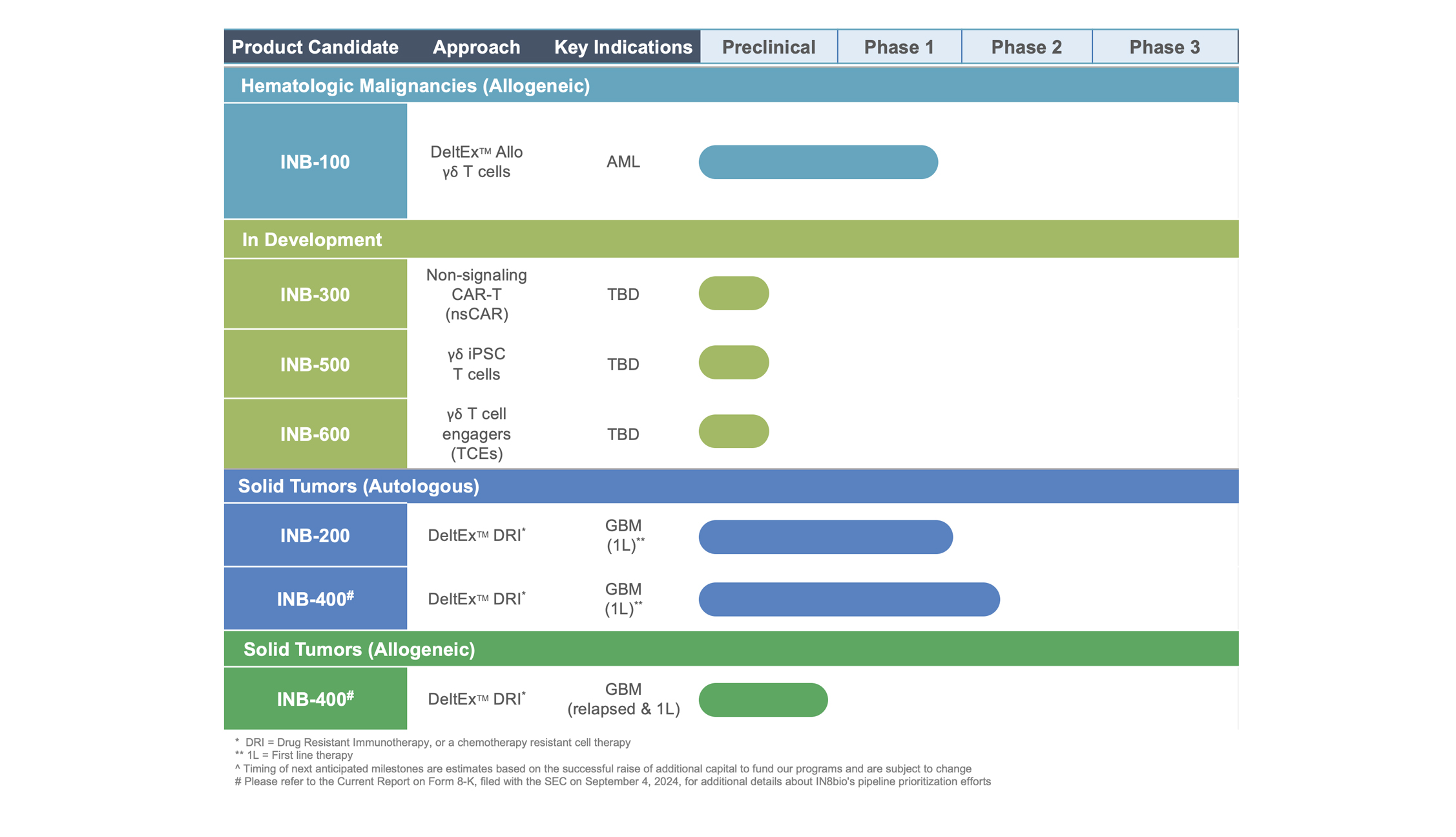
INB-200
DeltEx™ DRI Auto
INB-200, our DeltEx™ DRI Auto, is a genetically modified autologous gamma-delta T cell product candidate for the treatment of newly diagnosed GBM solid tumors. This was the first genetically engineered gamma-delta T cell therapy to be administered to patients. The Phase 1 repeat dose escalation clinical trial is being conducted at the O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham.

INB-100
DeltEx™ Allo
INB-100, our DeltEx™ Allo, is an allogeneic product candidate initially developed for the treatment of patients with acute leukemia undergoing hematopoietic bone marrow transplantation (HSCT). Our scientific founder, Dr. Lawrence Lamb, Ph.D., was the first to describe a survival benefit in HSCT patients with naturally elevated levels of gamma-delta T cells. INB-100 was designed to translate this observation into a treatment and provides donor derived allogeneic gamma-delta T cells to patients following their HSCT. We are conducting a Phase 1 dose escalation clinical trial at the University of Kansas Cancer Center.
INB-400
DeltEx™ DRI Auto and Allo
IINB-400, our DeltEx™ DRI Auto and Allo, is a product candidate in a Phase 2 multi-center clinical trial for the treatment of newly diagnosed glioblastoma, or GBM. This trial is currently enrolling and will assess autologous and allogeneic, genetically modified chemotherapy-resistant gamma-delta T cells in patients at specialized brain tumor centers across the United States. Since 2005, the standard of care treatment for GBM has been surgical resection followed by radiation and chemotherapy, referred to as the Stupp regimen. Most patients relapse in less than one year, with very few patients surviving beyond five years. INB-400 is engineered to be resistant to alkylating chemotherapy so it can be used in combination with the current standard-of-care Temozolomide (TMZ) to amplify immune signals, maximize tumor killing, and eliminate cancer cells.
INB-300
DeltEx™ nsCAR-T
INB-300 DeltEx™ nsCAR-T INB-300 is our DeltEx™ DRI non-signaling CAR (nsCAR) platform with several preclinical product candidates, including the INB-330 program targeting CD-33 for AML. Our nsCAR platform combines our expertise in gamma-delta T cells and genetic engineering. The nsCAR constructs lack signaling domains, which takes advantage of the unique properties of gamma-delta T cells to naturally differentiate between healthy and tumor tissues. The CAR is intended to enhance tumor cell targeting and recognition but killing only occurs through the natural gamma-delta T cell pathways that identify and respond to cellular stress. IN8bio is advancing new nsCAR constructs against multiple targets to treat both solid and hemtological tumors. This technology can also be combined with our chemotherapy resistance technology in DeltEx DRI nsCAR-T product candidates. This video demonstrates the cytotoxicity of our DeltEx nsCAR-T cells (clear) in vitro against cancer cells that express green fluorescent protein (green). Our nsCAR-T cells efficiently swarm, attack, and eradicate the tumor cells and illustrate the direct killing action of these gamma-delta T cells.
INB-500
iPSC Gamma-Delta T Cells
Our DeltEx platform has expanded its capabilities to include iPSC derived gamma-delta T cells. We were the first company to demonstrate the ability to generate both iPSC derived Vd1+ and Vd2+ gamma-delta T cell subtypes using cell-type specific differentiation processes. iPSC-derived gamma-delta cells could address existing challenges, including of donor sourcing and limitations of cell expansion in manufacturing. iPSCs, with their nearly unlimited self-renewal capacity and multi-lineage differentiation potential, offer the ability to to generate potentially ‘off-the-shelf’ therapeutic products for patients across a wide range of cancers.